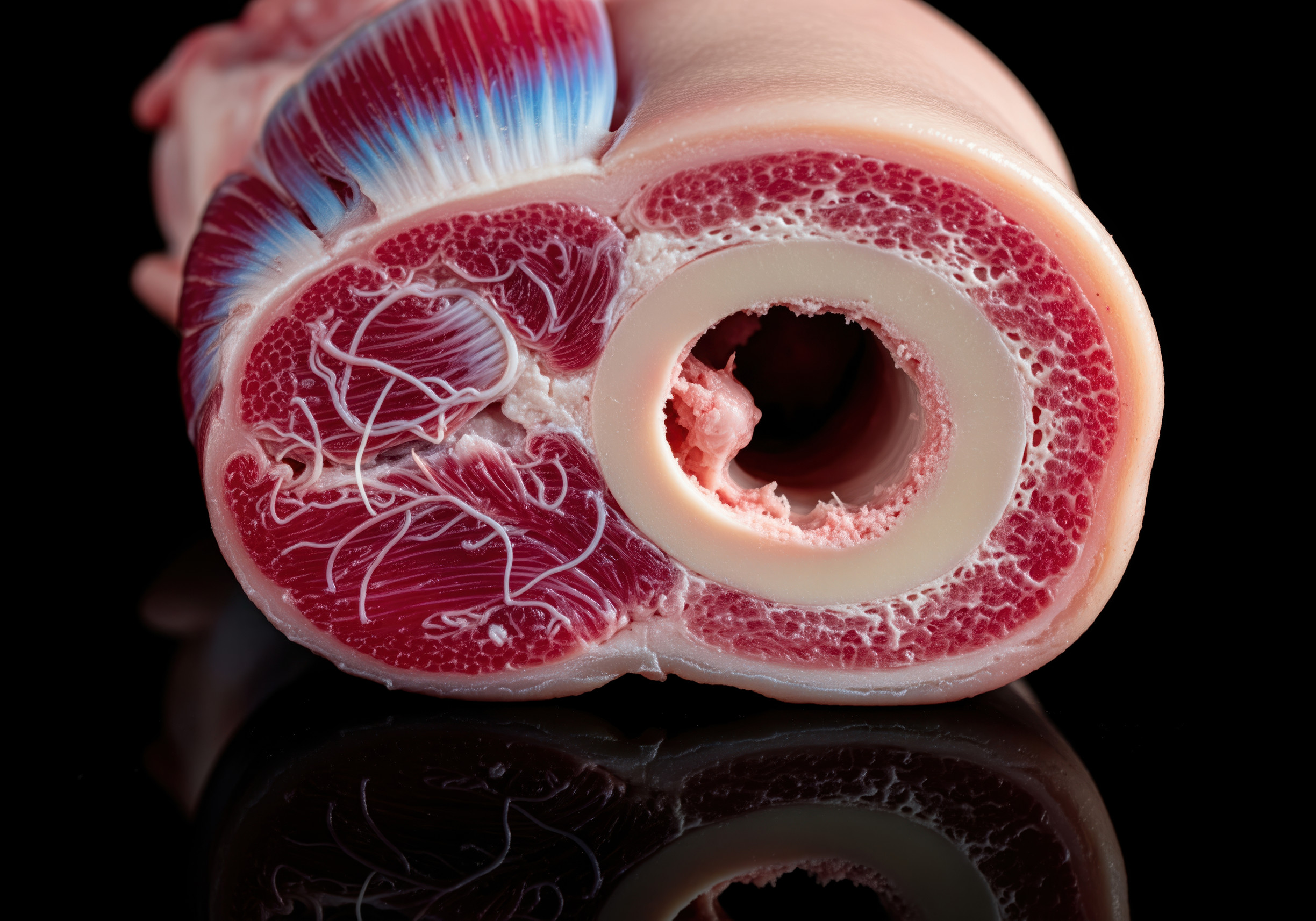
Our hearts are incredible for all that they do— but that said, they aren’t great at mending themselves. Consequently, after a heart attack, a lot of scar tissue forms in the heart, making it less pliable and unable to pump blood. Researchers have identified a way to reverse some of the damage caused by heart attacks in mice by transforming scar tissue into healthy tissue, which they say was inspired by how young hearts heal themselves.
Un-break our hearts
Scientists have made it a top priority to find ways to prevent and lessen the severity of cardiac events like heart attacks, which affect one person every 40 seconds in the United States alone. Although most research has focused on figuring out how to keep people from having heart attacks, more recent studies are looking into ways to heal damaged hearts, in particular the scar tissue that develops in the wake of a heart attack. Scar tissue that remains after a heart attack is much stiffer than normal cardiac tissue. Due to its decreased pliability, it poses a risk to the heart’s ability to pump blood effectively.
Earlier this year, scientists in Australia discovered that increasing elastin, the protein responsible for the elastic properties of several bodily tissues, could reduce cardiac scarring in rats. As the scars in the heart shrank and grew more flexible, the cardiac function returned to near-normal levels in the research.
Researchers at Duke University (DU) conducted the latest study, which analyzed the role of fibroblasts, cells critical in the development of both connective and scar tissue. Cellular reprogramming, a method that uses RNA, was supposed to be used to transform damaged fibroblasts into functional heart muscle after a heart attack. Previous research has examined the technique’s potential for use in a variety of contexts, including heart repair, motor function restoration in stroke patients, wound healing, and others.
In contrast to their findings with juvenile fibroblasts, researchers working with mice found that adult fibroblast cells were resistant to reprogramming.
They discovered that an oxygen sensor protein called Epas1 was responsible for the discrepancy, as it prevented the adult cells from reprogramming. The mature cells were able to successfully convert when Epas1 was blocked.
Becoming young again
More fibroblasts were transformed into heart muscle when the aging process was reversed, according to the study’s supervisor, Conrad Hodgkinson, an associate professor of medicine and pathology at the University of Colorado School of Medicine.
Researchers blocked Epas1 in mice who had experienced heart attacks by injecting RNA into the animals. The RNA was packaged in exosomes, which are sac-like structures present all over the body, and included the reprogramming instructions to transform fibroblasts into healthy heart tissue.
“Exosomes are kind of like shopping bags,” Hodgkinson explained. When a cell wants to convey a signal to nearby cells, it packs all its components into a bloated sphere. They allow cells to communicate with one another.
The method, to the delight of the team, worked as intended.
According to Hodgkinson, by reversing the aging of the fibroblasts in the heart, they “were able to recover almost all of the cardiac function lost after a heart attack.”
The researchers say their findings may have implications for other areas of medicine, such as the regeneration of neurons in the brain and the reversal of skin scarring in certain dermatological conditions because they used cellular reprogramming as a way to reverse the effects of aging on some cells.
Source study: Journal of Biological Chemistry— Neonatal and adult cardiac fibroblasts exhibit inherent differences in cardiac regenerative capacity
We are highlighting this piece as part of our annual “Best Of” roundup of articles published at The Optimist Daily this year. Today’s focus is on the top Science solutions of 2023.